Within the intricate world of steroid biochemistry, the study of 7-oxo-DHEA and its resulting metabolites stands at the forefront of analytical research. Among the most studied derivatives is Arimistane, a compound whose significance is rooted in both its unique structure and the detailed process by which it is formed. This article explores the degradation of 7-oxo-DHEA, the pathways that lead to metabolite formation—particularly Arimistane—and the critical role of analytical reagents in these processes. The content below is designed specifically for laboratory professionals, analytical chemists, and academic researchers.
Understanding 7-oxo-DHEA: A Biochemical Perspective
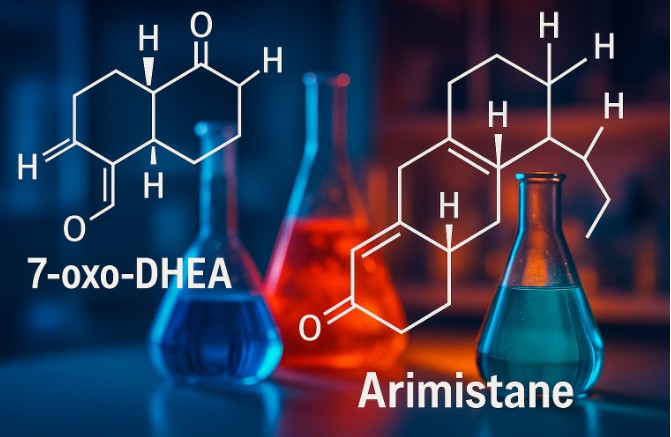
What is 7-oxo-DHEA?
7-oxo-Dehydroepiandrosterone (7-oxo-DHEA) is an oxygenated metabolite of the parent hormone DHEA, which itself is synthesized naturally in adrenal glands. 7-oxo-DHEA distinguishes itself by lacking androgenic properties, a feature that makes it especially valuable for research into metabolic and enzymatic processes, without interference from hormonal activities. The compound’s stability and reactivity also make it a preferred substrate for studies involving steroid degradation and metabolite mapping.
Research-Driven Applications
In research settings, 7-oxo-DHEA is commonly utilized for:
- Enzyme activity assays
- Metabolic stability profiling
- Comparative degradation studies
- Analytical method development using high-purity analytical reagents
Degradation Pathways: From 7-oxo-DHEA to Arimistane
Factors Influencing Degradation
The conversion of 7-oxo-DHEA into its metabolites—including Arimistane—is a multifaceted process, influenced by a range of environmental and experimental variables:
- pH & Buffer Systems: Acidic and basic buffers influence hydrolysis and oxidation, affecting the speed and products of degradation.
- Temperature: Elevated temperatures can accelerate molecular breakdown and drive reactions toward specific metabolites.
- Oxidative Environment: The presence of reactive oxygen species, peroxide, or specific oxidizing agents can modify degradation kinetics.
- Light Exposure: Ultraviolet and visible light can trigger photochemical reactions, leading to alternate metabolic byproducts.
- Solvent Systems: Solvents used for dissolution or extraction, as well as the choice of analytical reagent, can promote or inhibit certain pathways.
Stepwise Formation of Arimistane
The main transformation from 7-oxo-DHEA to Arimistane involves a reduction of the 7-oxo functional group, followed by a rearrangement and dehydrogenation process:
- Reduction of the 7-oxo group via enzymatic or chemical means
- Dehydrogenation at the C3 and C5 positions, resulting in a conjugated diene system
- Reconfiguration of the steroid skeleton to form Arimistane (Androsta-3,5-diene-7,17-dione)
Why Arimistane Matters in Research
Arimistane is not just a product of curiosity; its formation provides valuable insights into:
- Enzymatic reduction mechanisms
- Steroid biotransformation in non-biological systems
- The fate of exogenous steroidal compounds in laboratory studies
- The application of advanced analytical reagents to reliably identify low-abundance metabolites
Advanced Analytical Techniques for Steroid Profiling
High-Performance Liquid Chromatography (HPLC) & Ultra-Performance Liquid Chromatography (UPLC)
HPLC and UPLC are foundational in separating and quantifying 7-oxo-DHEA and its degradation products. These methods rely on:
- High-purity solvents as analytical reagents for reproducible results
- Gradient elution techniques for resolving closely related compounds
- Optimized column chemistry for steroid separation
Analytical reagents such as ion-pairing agents, pH buffers, and derivatization chemicals are key to enhancing detection sensitivity and chromatographic resolution.
Mass Spectrometry (MS/MS): Detection and Characterization
Mass spectrometry, especially in tandem (MS/MS) mode, is the gold standard for identifying unique fragmentation patterns of Arimistane and other metabolites.
- Electrospray Ionization (ESI) and Atmospheric Pressure Chemical Ionization (APCI) are two popular ionization methods, often improved by the addition of specific analytical reagents.
- Fragmentation libraries for steroidal metabolites provide reference spectra, improving identification confidence.
- Isotope-dilution techniques are used for accurate quantitation.
Nuclear Magnetic Resonance (NMR) and Infrared Spectroscopy (IR)
NMR and IR techniques are essential for:
- Confirming molecular structure
- Verifying the presence of key functional groups
- Differentiating between structural isomers
Proper sample preparation—using high-grade analytical reagents for purification and dissolution—is vital for acquiring high-quality spectral data.
The Critical Role of Analytical Reagent Selection
Every stage of 7-oxo-DHEA degradation and Arimistane identification hinges on the correct choice of analytical reagent. Whether it’s a derivatization agent to improve detectability, a solvent system to enhance extraction efficiency, or a buffer to stabilize intermediates, the quality and purity of reagents directly impact:
- Assay reproducibility
- Signal-to-noise ratio
- Limit of detection and quantification
- Sample stability and shelf-life
Categories of Analytical Reagents Used
- Buffer Systems: Maintain pH and ionic strength in chromatographic and spectrometric analyses
- Derivatizing Agents: Increase volatility for GC or improve UV response for HPLC
- Solvent Purity: Prevent background interference in sensitive assays
- Internal Standards: Allow accurate quantitation and normalization
Analytical Reagent choice is an overlooked but foundational element of every successful research workflow.
Validating Analytical Methods: Standards, Controls, and Quality
Reference Materials and Calibration
To ensure that observed metabolite formation—from 7-oxo-DHEA to Arimistane—is both accurate and reproducible, laboratories use:
- Certified reference materials (CRMs) for both the parent compound and its metabolites
- Calibration curves built with known concentrations for precise quantitation
Method Validation
A validated analytical method must demonstrate:
- Selectivity and specificity for the analyte of interest
- Linearity over the expected concentration range
- Precision and accuracy, evaluated through replicate analyses and spiked samples
- Stability studies to ensure samples and reagents do not degrade prior to analysis
Quality controls, including matrix-matched samples and blank runs, are incorporated into every batch to detect potential contamination or procedural errors.
Practical Laboratory Applications & Compliance
Arimistane and all other compounds referenced in this article are intended solely for laboratory and research use. Modern research facilities must operate within the guidelines set by regulatory bodies. This includes strict labeling, handling, and disposal practices for all analytical reagents and metabolites.
Disclaimer: These products are not intended for human or animal use, diagnosis, or treatment and should not be introduced into any in vivo system.
For further research insights, protocols, or to access analytical resources, visit goodnever.com.
Expanding Horizons: New Frontiers in Steroid Metabolite Analysis
Automation and High-Throughput Screening
With technological advancements, laboratories are increasingly adopting automated sample preparation and analysis workflows. These innovations allow:
- Processing hundreds of samples in parallel
- Reduced human error
- Improved consistency and reproducibility
Real-Time and Predictive Analytics
Emerging tools now allow researchers to monitor degradation reactions and metabolite formation in real time. Integration with AI and machine learning enables prediction of complex metabolic pathways, guiding reagent choice and experimental design.
Environmental and Forensic Applications
The study of steroid degradation isn’t limited to pharmaceutical or biochemical labs. Environmental scientists now monitor the breakdown of steroidal pollutants in soil and water, while forensic chemists analyze trace metabolites in toxicological screenings.
Frequently Asked Questions (FAQ)
Q1: What is the main reason to study the degradation of 7-oxo-DHEA?
A1: Understanding its degradation provides insight into metabolic pathways, enzyme actions, and analytical method performance for steroid profiling in laboratory research.
Q2: Why is Arimistane significant in metabolite studies?
A2: Arimistane represents a major stable metabolite, serving as a biomarker for 7-oxo-DHEA breakdown and as a standard for analytical method validation.
Q3: How important is the choice of analytical reagent in these studies?
A3: The selection of analytical reagent affects everything from extraction efficiency to detection sensitivity, making it critical for reproducibility and reliability.
Q4: Can these findings be used for therapeutic or diagnostic purposes?
A4: No, all findings and materials discussed are for laboratory research use only and are not approved for any human or animal application.
Conclusion
The journey from 7-oxo-DHEA to Arimistane illustrates the complexity and scientific importance of steroid metabolite analysis. Through the careful application of modern chromatographic, spectrometric, and spectroscopic methods—and the strategic use of high-purity analytical reagents—researchers are uncovering new pathways and setting higher standards for accuracy and reliability.
Whether for method development, environmental analysis, or academic exploration, understanding these degradation mechanisms will continue to drive innovation in analytical chemistry. As laboratories seek greater sensitivity and reproducibility, the choice of Arimistane and the right analytical reagent will remain central to meaningful scientific advancement.